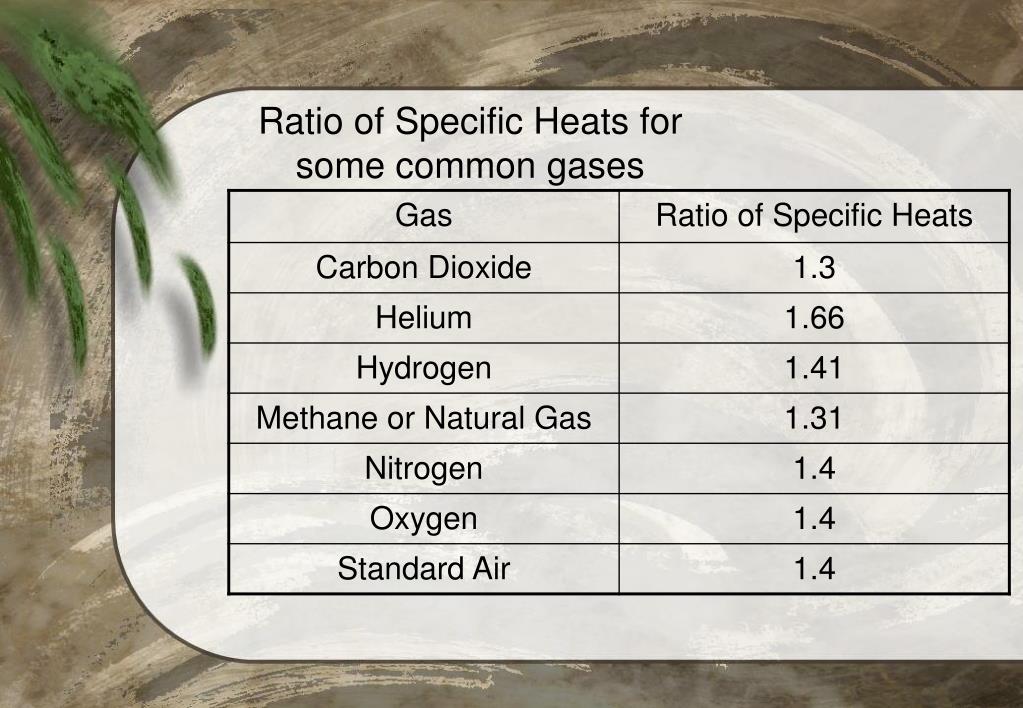
What Is Heat Capacity Air . air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon, and various. with increasing temperature the heat capacity slightly increases due to exciting of the vibrational degrees of freedom in the oxygen. Heat capacity is an extensive property of matter, meaning it is proportional to the size of. specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. specific heat of air is 1006 j/g k. The nominal values used for air at 300 k are c p = 1.00 kj/kg.k, c v = 0.718 kj/kg.k,, and k = 1.4. This formulation is valid for. specific heat capacities of air.
from www.slideserve.com
specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Heat capacity is an extensive property of matter, meaning it is proportional to the size of. This formulation is valid for. with increasing temperature the heat capacity slightly increases due to exciting of the vibrational degrees of freedom in the oxygen. specific heat of air is 1006 j/g k. The nominal values used for air at 300 k are c p = 1.00 kj/kg.k, c v = 0.718 kj/kg.k,, and k = 1.4. air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon, and various. specific heat capacities of air.
PPT Determining the Specific Heat Capacity of Air PowerPoint
What Is Heat Capacity Air with increasing temperature the heat capacity slightly increases due to exciting of the vibrational degrees of freedom in the oxygen. air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon, and various. with increasing temperature the heat capacity slightly increases due to exciting of the vibrational degrees of freedom in the oxygen. Heat capacity is an extensive property of matter, meaning it is proportional to the size of. This formulation is valid for. specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. The nominal values used for air at 300 k are c p = 1.00 kj/kg.k, c v = 0.718 kj/kg.k,, and k = 1.4. specific heat of air is 1006 j/g k. specific heat capacities of air.
From www.slideserve.com
PPT Advance Chemical Engineering Thermodynamics PowerPoint What Is Heat Capacity Air The nominal values used for air at 300 k are c p = 1.00 kj/kg.k, c v = 0.718 kj/kg.k,, and k = 1.4. air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon, and various. specific heat of air is 1006 j/g k. with increasing temperature the. What Is Heat Capacity Air.
From www.slideshare.net
Heat Capacity What Is Heat Capacity Air specific heat of air is 1006 j/g k. with increasing temperature the heat capacity slightly increases due to exciting of the vibrational degrees of freedom in the oxygen. This formulation is valid for. Heat capacity is an extensive property of matter, meaning it is proportional to the size of. air is a mixture of gases, 78% nitrogen. What Is Heat Capacity Air.
From www.slideserve.com
PPT First Law of Thermodynamics PowerPoint Presentation, free What Is Heat Capacity Air The nominal values used for air at 300 k are c p = 1.00 kj/kg.k, c v = 0.718 kj/kg.k,, and k = 1.4. Heat capacity is an extensive property of matter, meaning it is proportional to the size of. air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon,. What Is Heat Capacity Air.
From www.slideserve.com
PPT Chapter 17 Thermochemistry PowerPoint Presentation, free What Is Heat Capacity Air This formulation is valid for. air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon, and various. The nominal values used for air at 300 k are c p = 1.00 kj/kg.k, c v = 0.718 kj/kg.k,, and k = 1.4. with increasing temperature the heat capacity slightly increases. What Is Heat Capacity Air.
From www.tec-science.com
Specific heat capacity of gases (at constant volume or pressure) tec What Is Heat Capacity Air air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon, and various. specific heat of air is 1006 j/g k. The nominal values used for air at 300 k are c p = 1.00 kj/kg.k, c v = 0.718 kj/kg.k,, and k = 1.4. specific heat (c) is. What Is Heat Capacity Air.
From www.researchgate.net
Specific heat capacity of air as a function of temperature What Is Heat Capacity Air specific heat capacities of air. This formulation is valid for. specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. with increasing temperature the heat capacity slightly increases due to exciting of the vibrational degrees of freedom in the oxygen. Heat capacity is an. What Is Heat Capacity Air.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow What Is Heat Capacity Air Heat capacity is an extensive property of matter, meaning it is proportional to the size of. specific heat capacities of air. specific heat of air is 1006 j/g k. This formulation is valid for. air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon, and various. The nominal. What Is Heat Capacity Air.
From www.slideserve.com
PPT Chapter 17 Thermal Properties PowerPoint Presentation, free What Is Heat Capacity Air air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon, and various. specific heat capacities of air. This formulation is valid for. Heat capacity is an extensive property of matter, meaning it is proportional to the size of. specific heat (c) is the amount of heat required to. What Is Heat Capacity Air.
From www.geeksforgeeks.org
Heat Capacity Definition, Formula, Unit, Examples, FAQs What Is Heat Capacity Air The nominal values used for air at 300 k are c p = 1.00 kj/kg.k, c v = 0.718 kj/kg.k,, and k = 1.4. air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon, and various. This formulation is valid for. specific heat of air is 1006 j/g k.. What Is Heat Capacity Air.
From www.slideserve.com
PPT Determining the Specific Heat Capacity of Air PowerPoint What Is Heat Capacity Air This formulation is valid for. specific heat of air is 1006 j/g k. air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon dioxide, argon, and various. with increasing temperature the heat capacity slightly increases due to exciting of the vibrational degrees of freedom in the oxygen. specific heat. What Is Heat Capacity Air.
From www.differencebetween.com
Difference Between Heat Capacity and Specific Heat Compare the What Is Heat Capacity Air specific heat capacities of air. with increasing temperature the heat capacity slightly increases due to exciting of the vibrational degrees of freedom in the oxygen. specific heat of air is 1006 j/g k. This formulation is valid for. Heat capacity is an extensive property of matter, meaning it is proportional to the size of. specific heat. What Is Heat Capacity Air.
From physicsomets.netlify.app
Physics Formula For Specific Heat Capacity What Is Heat Capacity Air The nominal values used for air at 300 k are c p = 1.00 kj/kg.k, c v = 0.718 kj/kg.k,, and k = 1.4. specific heat of air is 1006 j/g k. specific heat capacities of air. Heat capacity is an extensive property of matter, meaning it is proportional to the size of. This formulation is valid for.. What Is Heat Capacity Air.
From haipernews.com
How To Calculate Specific Heat Capacity Bbc Bitesize Haiper What Is Heat Capacity Air specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. specific heat capacities of air. with increasing temperature the heat capacity slightly increases due to exciting of the vibrational degrees of freedom in the oxygen. specific heat of air is 1006 j/g k.. What Is Heat Capacity Air.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube What Is Heat Capacity Air Heat capacity is an extensive property of matter, meaning it is proportional to the size of. The nominal values used for air at 300 k are c p = 1.00 kj/kg.k, c v = 0.718 kj/kg.k,, and k = 1.4. This formulation is valid for. specific heat of air is 1006 j/g k. specific heat (c) is the. What Is Heat Capacity Air.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID3150253 What Is Heat Capacity Air Heat capacity is an extensive property of matter, meaning it is proportional to the size of. This formulation is valid for. with increasing temperature the heat capacity slightly increases due to exciting of the vibrational degrees of freedom in the oxygen. air is a mixture of gases, 78% nitrogen and 21% oxygen with traces of water vapor, carbon. What Is Heat Capacity Air.
From gamesmartz.com
Heat Capacity Definition Easy to Understand What Is Heat Capacity Air specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Heat capacity is an extensive property of matter, meaning it is proportional to the size of. with increasing temperature the heat capacity slightly increases due to exciting of the vibrational degrees of freedom in the. What Is Heat Capacity Air.
From www.slideshare.net
Heat Capacity What Is Heat Capacity Air The nominal values used for air at 300 k are c p = 1.00 kj/kg.k, c v = 0.718 kj/kg.k,, and k = 1.4. specific heat of air is 1006 j/g k. with increasing temperature the heat capacity slightly increases due to exciting of the vibrational degrees of freedom in the oxygen. specific heat capacities of air.. What Is Heat Capacity Air.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow What Is Heat Capacity Air specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. The nominal values used for air at 300 k are c p = 1.00 kj/kg.k, c v = 0.718 kj/kg.k,, and k = 1.4. air is a mixture of gases, 78% nitrogen and 21% oxygen. What Is Heat Capacity Air.